Here at the Science Museum of Virginia, we’ve been exploring all things vaccine through our blogs, Instagram swipe series, Lunch Break Science presentations, and more.
We’ve reviewed the phases of human trial testing previously in blog and Instagram formats, but we wanted to do a deeper dive. So, what are human vaccine trials really like and who conducts them?
To answer that question and help dispel some of the mystery involved in drug trials, our Scientist in Residence Dr. Catherine Franssen paid a visit to Dr. Robert Call, President and Medical Director of Clinical Research Partners here in Richmond. Here’s what she learned about the vaccine testing process.
Clinical Research Partners has several locations, and on a recent Saturday morning I visited the one in Henrico’s West End. It felt like visiting a doctor’s office, except I also got a behind-the-scenes look at the big open room with desks and hundreds of files that serves as mission control for Dr. Call.

Image credit: Getty Images
Dr. Call is a top allergist, working full time with Richmond Allergy and Asthma Specialists seeing patients. He’s also run more than 250 clinical research trials of studies on drugs and vaccines. Over the past decade, he and Clinical Research Partners Chief Executive Officer Annette Bennett have made Richmond an important research hub. Recently Dr. Call has been spending his time on the AstraZeneca COVID-19 vaccine research.
Despite his long experience with clinical research, Dr. Call told me that his involvement in this trial is special. He says that being a part of this effort has made him feel like he’s part of something really big, and feels like he’s helping save the world. He conveys that rare and wonderful feeling of being in the right place at the right time, and genuinely making a difference.
From our conversations as we walk around, it’s obvious that Dr. Call is putting in significant hours on this study and that he is excited and energized by this work. As he strolls the halls of the medical office, he greets each doctor, nurse, patient coordinator, and other staff members by name, often introducing me while singing the praises of the many people setting up their stations to start a day of seeing participants through a research trial. He feels connected to his team, and humbly refers to himself as the head cheerleader as they do the bulk of the work.
We rarely think about how isolating it can feel for doctors – who have chosen a profession of caring for others – to see patients briefly on most days, interacting with nurses and staff only minimally, sometimes spending more time on paperwork than with people. Being involved in a research project allows a doctor to feel part of a team in a different way. Dr. Call clearly feels like this current effort is a passion project that rejuvenates him.
"Without volunteers there is no clinical research, and without clinical research there are no new drugs."
Dr. Robert Call
Clinical Research Partners in Richmond was selected to be one of the few locations in the USA to run the AstraZeneca COVID-19 vaccine trial due to a positive track record in many other drug trials for high participation and for the diversity of people they are able to recruit.
Dr. Call walked me through the entire process step by step. Each drug trial is a little bit different, but there are a lot of similarities between the COVID-19 vaccine research and many other trials he’s worked on in the past.
Step 1: Recruit volunteers
Clinical Research Partners recruits volunteers to participate in the vaccine study through ads, their website, word of mouth, and a lot of phone calls. As with most research trials, volunteers will be paid a nominal fee, and the ethics of that payment are reviewed as thoroughly as the rest of the research protocols.
Many of the team members have other jobs and work on this research part time or temporarily. For example, the person heading up recruitment on this study is an insurance salesperson, which seems to be a perfect skill set since Clinical Research Partners led the world in clinical trial enrollments!
Step 2: Get volunteers into the research center
There’s a big step between someone agreeing to take part in a vaccine trail and actually getting that person to show up at the research facility. But, once they do, check-in at the front desk is typical of any medical office these days: get your temperature taken and receive a clipboard with a pile of forms to fill out.
Step 3: Informed consent
Getting informed consent from a participant is the most important step according to Dr. Call. This is absolutely essential and can take some time. There can be up to 50 pages of details, and the volunteer must go through and initial every page with a patient coordinator present to answer questions and review any concerns. Volunteers are given information about the study and presented with potential risks. They are assured that the drug company will not receive their names or identifying information, they will simply be coded as initials, a number, and usually the age and sex for study purposes.
Sometimes volunteers sometimes opt out at this point because the details become overwhelming. This part often happens in a room with a desk, before moving on into the exam room.
Step 4: The protocol
This is the part where the volunteer actually gets the jab: the vaccine or a placebo (a non-medicated substance used as a control). But there’s more to it than that!
While in the medical exam room, the volunteer will undergo a physical examination and preliminary assessments.
- First a nurse takes vital signs and a doctor performs a comprehensive medical examination, which is fairly typical of a routine checkup.
- Then, depending on the specific study that’s being conducted, there will be some other preliminary tests, which the volunteer will have been told about in step three. A nurse may take some bloodwork. There may be questionnaires about how the volunteer is feeling or a mental health scale if they are concerned about a side effect such as anxiety or depression. If the volunteer is participating in a drug trial for a certain disease or condition, like eczema, there will be a specific way of assessing the patient’s condition, like an eczema scale to rate the severity of the condition in that patient so the medical professionals can follow up in the future and see how much it improves. These are all the baseline tests.
Once all the information has been collected, and the research group affirms that the volunteer is fit for the trial, there are two more parts of step four: randomization and treatment.
- The volunteer’s file is walked down to the end of the hall to the pharmacy. A pharmacist and pharmacy techs are in a windowless room with multiple signs posted outside to warn all other personnel to stay out because they might accidentally learn which treatment a patient is given. In this room, the study is blinded. When volunteer information comes in, a computer randomizes the study group. The pharmacy workers prepare a syringe – either vaccine or placebo – labelled with the volunteer’s ID information (but not what it is) and place it on a tray on a medical cart outside the room. A nurse picks up the tray and injection, and confirms the identity of the volunteer, but does not know what’s in the injection. This is key to the double blind experimental design.
- Lastly, the nurse administers the injection.
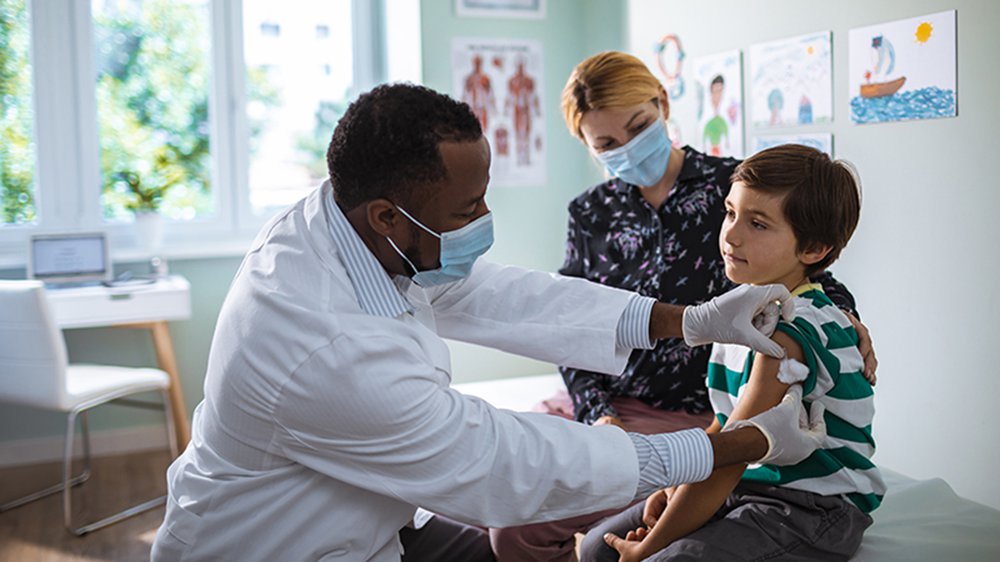
Image credit: Getty Images
Step 5: Observation
Volunteers sit in an open waiting area observation lounge for 15 minutes after receiving the injection. The lounge is near the nurses station so they monitor any responses to the vaccine. The most important mission in clinical research is subject safety, even more so than the trial outcome.
Step 6: Check out
Instead of paying your bill at checkout like at a doctors visit, this is the other way around: volunteers get paid a small fee for their participation. Typically they are issued a reloadable payment card that can be refilled online through a centralized system as they complete phases of the trial.
Step 7: Follow up
A centralized system organizes weekly follow-up phone calls to all participants to check their symptoms. The researchers are primarily interested if there has been any reaction to the shot or if the participant has tested positive for COVID-19 since receiving the vaccine, but also want to make note if the trial participant has had any other adverse events, like a twisted ankle. Keeping track of any and all adverse events is important to determine if they will have an impact of the vaccine's effectiveness and/or if there are patterns that develop within reported adverse events.
The COVID-19 vaccine follow-ups are fairly straightforward, but Dr. Call shared with me some of the different kinds of research follow-ups that can happen. Some studies ask volunteers to keep diaries as it’s easier to remember all the details if they are tracked throughout the week instead of having to remember everything during the weekly phone call.
For some research studies, volunteers take home a special armband for monitoring heart rate and blood pressure. It can hook into the computer and send the research team data through an internet portal. This can be done with researchers blind, or unaware, to who the patient is as the information is identified by only their assigned number.
After all the follow-up calls are complete, Dr. Call gets to do the final outreach to tell the participant if they had the COVID-19 vaccine or the placebo, to “unblind” them. When I visited, he was working through calling large batches of the 1,800 participants now finished with the study.
Whichever injection they received, the participant contributed greatly to the vaccine safety testing, and helped move the vaccine one step closer to approval. Without volunteers there is no clinical research, and without clinical research there are no new drugs.
Clinical Research Partners is wrapping up the AstraZeneca vaccine research, and that vaccine – the third for COVID-19 – is already in use in several countries. It is expected to be approved for emergency use in the USA in the coming months.
Need vaccine? Learn how to get your shot at Vaccinate.Virginia.gov or call 1-877-VAX-IN VA. 8am - 8pm. Language translation available TTY users dial 7-1-1.
¿Necesitas vacunarte? Enterate como conseguir tu vacuna Vaccinate.Virginia.gov o llamando al 1-877-829-4682 de 8am a 8pm. Traduccion disponible en tu idioma. Usuarios de TTY pueden marcar al 7-1-1.


