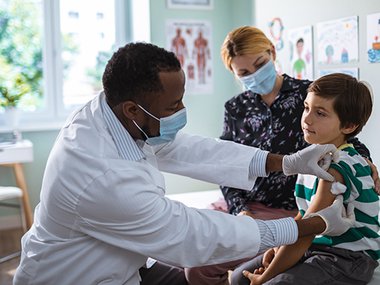
On November 2, 20201, the Centers for Disease Control and Prevention (CDC) endorsed the recommendation that children 5 to 11 years old be vaccinated against COVID-19 with the Pfizer-BioNTech pediatric vaccine. With that news, we wanted to revisit some of the information shared recently at our Kids & COVID-19 Q&A.
You can watch Dr. Danny Avula, Director of the Richmond City and Henrico County Health Departments, answer many of these questions in the event recording featured on our YouTube page. Our Life Scientist, Dr. Catherine Franssen, who moderated the question-and-answer session, has put together this summary of the discussion with information from trusted professionals and resources in science and medicine.
The free, virtual event is part of the Communities for Immunity initiative. Communities for Immunity is made possible with funding from the Centers for Disease Control and Prevention and the Institute of Museum and Library Services. For more information, visit www.communitiesforimmunity.org.
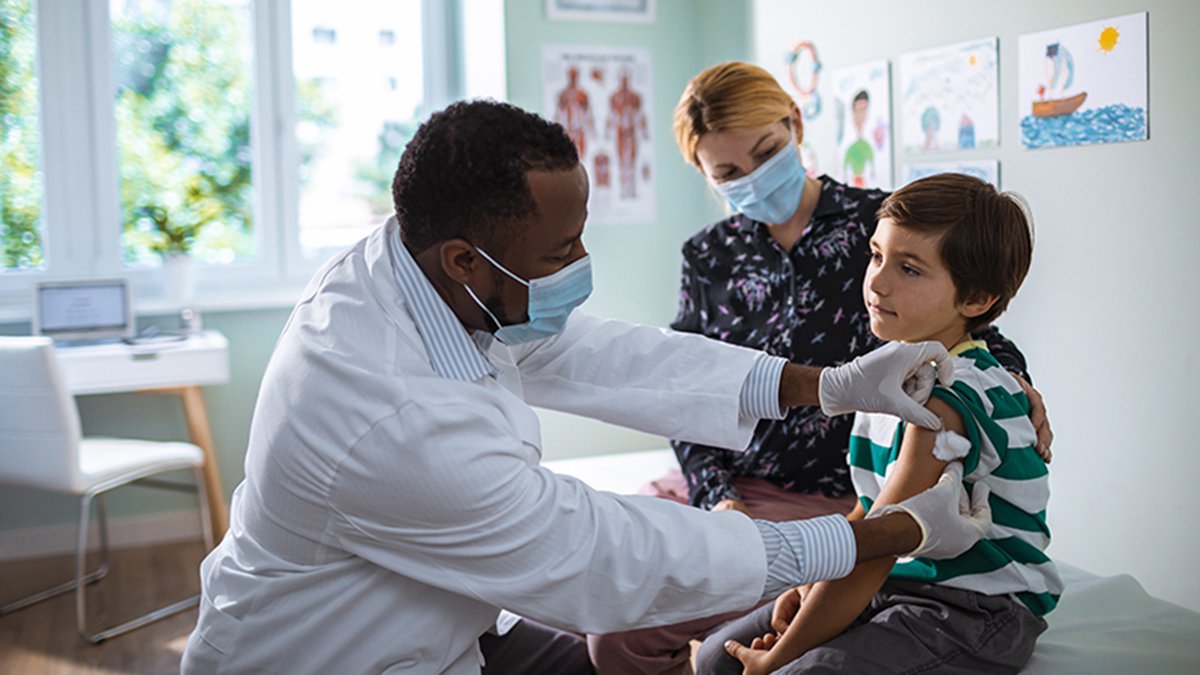
If children have a lower risk from a COVID-19 infection, why should they get the vaccine?
- Children make up more than a fourth of new COVID-19 cases in the United States.
- Although many children have mild disease, some kids are hospitalized, and some die. COVID-19 infection is now the eighth leading cause of death in the United States for children.
- Children are at significant risk for long-term health problems from a Delta variant infection. Some children have had long-term difficulty breathing and thinking, and cannot return to normal activities long after their COVID-19 infection.
- Kids can spread the virus to others including vulnerable members of their family and community. Vaccination helps to reduce spread.
- Children do better academically and developmentally with in-person learning. There have been more than 2,000 school closures due to COVID-19 infections. Vaccination will help to stop outbreaks and keep schools open.
- The vaccine is a safer alternative to risking COVID-19 virus infection, and the American Academy of Pediatrics strongly encourages it for all children.
My child has had COVID-19. Do they still need the vaccine?
Yes. Some people who have had a COVID-19 infection can get reinfected. Giving a messenger RNA (mRNA) vaccine (Pfizer or Moderna) after a COVID-19 infection boosts a person’s immunity, thus protecting them against reinfection.
The vaccine should be given to the child after they have recovered, are symptom-free and their quarantine period is over.
What are the long-term side effects of the vaccine?
Millions of adults and older children have gotten the Pfizer vaccine, as have thousands of children ages 5 to 11, without major side effects.
mRNA in the vaccine is broken down and eliminated by the body within a few days so it cannot have long-term effects. mRNA never goes into the cell nucleus where our DNA is located, so it cannot change or influence our genes. It’s also not stored within organs or tissue, so it will not reappear in the body as a child ages.
Historically, we know that if a vaccine will have a serious side effect, it will be seen within the first two months of its use. The other inactive ingredients in the vaccine have been used safely in other medications and in foods for many years.
For a more detailed outline of long-term effects, please visit https://smv.org/learn/blog/are-there-any-long-term-effects-covid-19-vaccine/.
Should I wait until my 11 year old turns 12 to get the higher dose?
Vaccine doses are given by a child’s age, not their weight. A large 11 year old and a small 11 year old have similar immune systems and need the same dose.
Are there children who shouldn’t get the COVID-19 vaccine?
No. According to the American Academy of Pediatrics, all children, and especially those with medical issues, should be vaccinated against COVID-19.
Will the vaccine make my child sick?
The COVID-19 vaccine could cause soreness in the arm in which the shot was given, fatigue, chills, fever and headaches. These subside within a few days.
Could the COVID-19 vaccine affect my child’s fertility?
There is no evidence of infertility related to COVID-19 antibodies produced as a result of the vaccine, and no evidence that the vaccine could affect future fertility.
Regarding menstrual changes, a small number of women who got the vaccine and a small number of women who got the virus have both reported menstrual changes that are temporary.
Animal studies and human clinical trials show no reproductive harm from the mRNA vaccines. Pregnant women have gotten the vaccine safely, thus protecting their unborn child. Millions of people of reproductive age have gotten the vaccine without fertility issues.
To dive into the vaccine’s impact on sexual health, please visit https://smv.org/learn/blog/what-effects-will-covid-19-vaccine-have-sex-reproduction-and-fertility/.
Can a child get another vaccine and a COVID-19 vaccine at the same time?
Yes.
What about the risk of heart inflammation?
Vaccine-related myocarditis, or inflammation of the heart, is very different from getting classic viral myocarditis.
Vaccine myocarditis has been seen rarely in teenage boys, is mild, treated with ibuprofen and resolves after a couple of days.
In contrast, viral myocarditis from a COVID-19 infection can cause problems with how the heart works long term. COVID-19 infection also causes myocarditis at much higher rates than the vaccine. Getting vaccinated protects you from COVID-associated myocarditis.
The COVID-19 vaccine was made so quickly. How do I know it’s safe for my child?
The COVID-19 vaccine was developed faster than other vaccines and drugs due to drastic time savings at each step in the process.
Research was already underway by several companies and universities studying other coronaviruses, which have been known for 20+ years. When COVID-19 emerged, the genomic sequence was worked out quickly and shared publicly. That information is usually a trade secret and results in delays for anyone who doesn’t have it. Also, since there was ample funding in the development of the COVID-19 vaccine due to purchase guarantees, companies could do tests in the pre-clinical and clinical phases at the same time.
For a step-by-step review of the time-saving process, please visit https://smv.org/learn/blog/question-your-world-how-did-covid-vaccine-get-made-so-quickly/.
A full transcript from the October 14 Kids & COVID-19: A Vaccine Q&A with Dr. Danny Avula is available here.