If you’ve been to the cardiologist recently, they have possibly reminded you that a little less salt would be good for you.
Much like your doctor advocating for your heart, we’re here to advocate for less salt on your roads, sidewalks and driveways — because it’s not just the roads you’re salting.
During the winter, salts are the most commonly used deicing agents — keeping our streets and sidewalks safe from ice and snow. When a salt (like sodium chloride, magnesium chloride or calcium chloride) dissolves in water, it lowers the freezing point, preventing or melting ice below the usual 32 degrees Fahrenheit. By reducing the amount of dangerous ice on the roads, salts undoubtedly prevent injury and save lives. Studies show that salts can reduce road collisions by around 85% depending on the weather conditions. In the United States, road salt usage has more than doubled since 1975. While safety is a top priority, when salt is overapplied it can cause negative effects to the environment, corrode infrastructure and contaminate our drinking water.
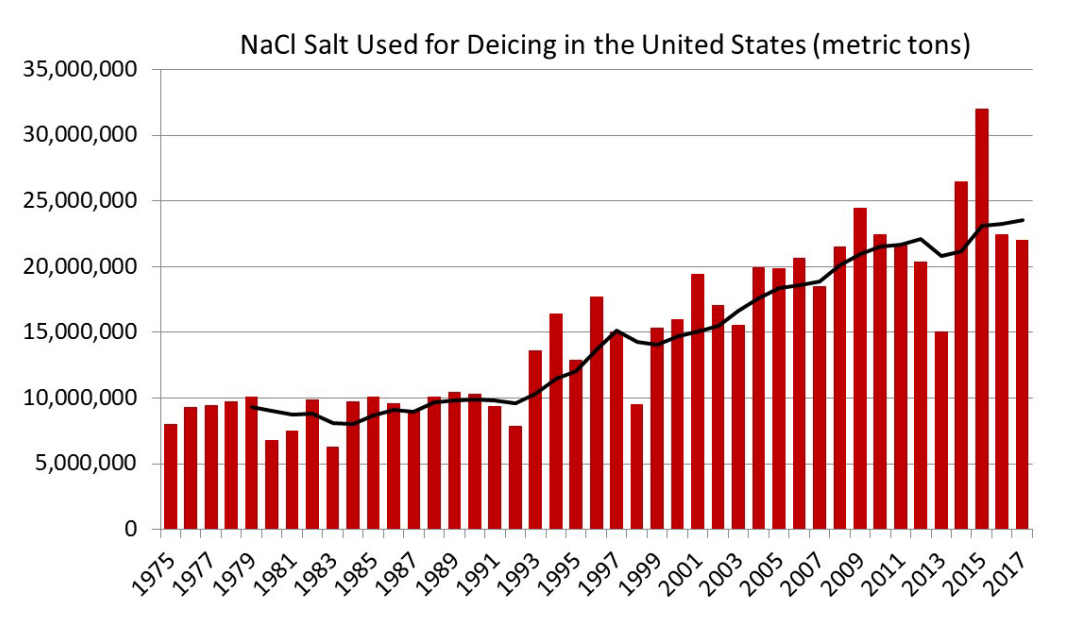
(Source: US Geological Survey, 2017, Salt statistics, in Kelly, T.D., and Matos, G.R., comps., Historical statistics for mineral and material commodities in the United States: US Geological Survey Data Series 140)
When it rains throughout the year, water runs off paved surfaces like roads, sidewalks and parking lots, carrying pollutants such as fertilizers, oils and pet waste into local bodies of water. Due to increased use of road salt in the colder months, many rivers show increases in chloride pollution.
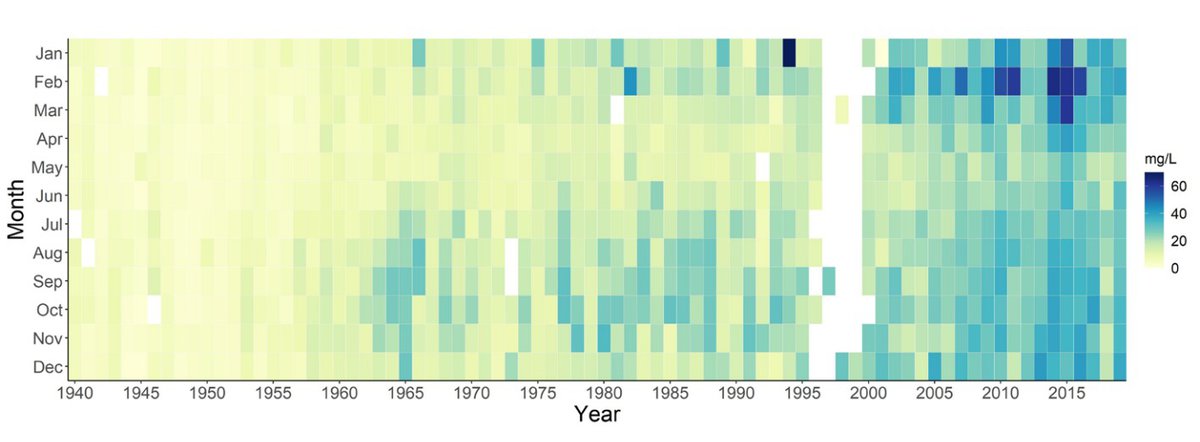
Chloride concentrations have risen almost 10-fold in the past 80 years in the Potomac River at Great Falls. (Source: Interstate Commission on the Potomac River Basin)
Road salt pollution is the leading cause of chloride pollution in U.S. waterways. Once salt is dissolved into the environment, there is no feasible way to remove it. As a result, it takes only 1 teaspoon of salt to permanently pollute 5 gallons of water. That means those common 50-pound bags of salt at the hardware store can permanently pollute 10,000 gallons of water each!
Plants and animals that live in freshwater habitats can’t all survive in saltier conditions. Some larger organisms may be less sensitive to changes in salinity, while the smaller organisms they rely on for food are more sensitive. These smaller organisms, like underwater insects and crustaceans, not only provide food for larger organisms in their habitat, they also help control algae.
Chloride, an ion present in most road salts, is corrosive and deteriorates pipes and other infrastructure. While the direct cost of road salt is relatively low when purchased, there are significant indirect costs associated with the damage it can cause — around $16-19 billion each year. Because salt cannot be removed at most water treatment facilities, homes with lead or copper pipes are at higher risk of lead corrosion in their tap water when salt pollution is present.
How can you take action?
- Shovel walkways before the snow turns to ice. If shoveling is too difficult, consider putting down a tarp before the snow. Then, simply remove the tarp, dumping the snow off to the side.
- Only use a mug’s worth (12 oz) of salt to treat 10 sidewalk squares or a 20 ft driveway. You do not need to feel the crunch under your feet for salt to do its job. Putting more salt on a surface does not make snow and ice melt faster or eliminate the need for plowing or shoveling.
- Sweep up excess salt and reuse it after the hazards have passed.
- Use a brine application, which mixes salt with water and is sprayed on surfaces.
- Help track salt pollution in your area for free by joining Salt Watch.
- Take photos and report excess salt.
- For VDOT roads, report excess salt to VDOT’s website.
- For city or county roads, report to your local 311.

Reducing the amount of salt you’re using isn’t just good for your heart — it’s good for your environment, too!


